Different coatings
Tablets can either be ‘sugar coated’, ‘film coated’, ‘enteric coated’ or coated to modify how the drug is released into the body (modified release). Each of these coatings are there for different reasons and it is important to understand these reasons before deciding whether it is safe or appropriate to crush tablets.
This page explains why the coat may be on the tablet and what it means if you choose to crush or place the tablet in water to dissolve before swallowing.
Film and sugar coating
A sugar coating is basically is a thick, hard coating of sugar surrounding the tablet. It is no different in design to the sugar coatings placed on Smarties® or Minstrels ®. This is a traditional method used to hide the flavour of particularly unpleasant tasting drugs e.g. ibuprofen and quinine, both of which are very bitter. The other advantage of a sugar coating is that it can prevent light or moisture from entering the tablet, which causes the drug to break down too quickly.
Due to the increase in tablet size caused by sugar coating, drug manufacturers have largely changed to using ‘film coatings’. These are very thin layers of a safe ingredient placed around the tablet to again protect the tongue from the flavour of the contents and protect the contents from moisture and light. The film will break down with agitation and significant amounts of moisture (saliva or stomach acid) and therefore does not significantly affect the way in which the drug is absorbed into the body.
Crushing these tablets therefore may not seriously effect how the drug is released but may cause the resultant mixture to be unpleasant to taste.
Enteric coating
If a tablet is described as having an ‘enteric coating’ (e/c) or ‘gastro-resistant’, it means that there is a coating which is designed to hold the tablet together when in the stomach. This clever science relies on the fact that the stomach is acid and the intestines, where food goes after the stomach, are not. The coating is designed to hold together in acid conditions and break down in non-acid conditions and therefore release the drug in the intestines.
There are three reasons for putting such a coating on a tablet or capsule ingredient:
- To protect the stomach from the drug
- To protect the drug from the stomach
- To release the drug after the stomach e.g. in the intestines
The drugs which most commonly cause stomach ulcers like aspirin, diclofenac and naproxen are frequently available with enteric coatings. Omeprazole, which is a drug which stops the stomach from producing acid, is itself broken down in acid and therefore the drug generally has an enteric coating around it either as a granule in the capsules or as a granule in the dispersible form. Sulfasalazine is used either for the treatment of arthritis or for the treatment of Crohn’s disease which is inflammation of the intestines. When used for arthritis, it is very often given without an enteric coating so that it can be absorbed more quickly. For Crohn’s, it is needed to work in the intestines so it is given an enteric coating.
It can be seen that an enteric coating has advantages and therefore such tablets or the contents of enteric coated capsules should never be crushed before being taken.
Modified release
‘Modified release’ means that the escape of the drug from the tablet has been modified in some way. Usually this is to slow the release of the drug so that the medicine does not have to be taken too often and therefore makes it easier to remember to take. The other benefit from modifying release is that the concentration of the drug in the body goes up slowly, is less likely to go very high and therefore reduces the chance of side effects.
Tablets and capsules which are designed to provide modified release often have the letters MR, LA, XL, CR or SR in their names e.g. Diffundox MR, Elantan LA, Dilzem XL Calcicard CR, Dilcardia S. Sometimes the words ‘slow’ or ‘retard’ can be used to denote modified release e.g. Diclomax retard, Voltarol retard & Slow K.
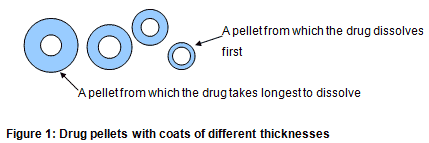
There are a number of ways in which a medicine can have its release modified. Perhaps the most famous is that used in Contac 400 capsules. The pellets inside are of different thicknesses and therefore the thinnest release the drug first and the thickest last (Figure 1).
Another method used is to put the drug in a viscous liquid which breaks down slowly itself and therefore releases the drug slowly. One method which has been tried in the past has been to put a non-dissolving coating around the tablet or capsule, laser a small hole in it and then let the drug only release through the hole. In such cases, patients frequently report passing the tablet or capsule whole and worrying whether it is actually working. Frequently, they are just passing the outer coat in their stools as this is how the medicine is designed to work and the drug has long been absorbed.
Modified release products usually have a higher than normal amount of the drug within them and therefore if they are crushed, the whole dose will be released very quickly and could be dangerous. Modified release products should never be crushed or modified before being taken.